Stay updated on BMS-986226 Immunotherapy Combo Clinical Trial
Sign up to get notified when there's something new on the BMS-986226 Immunotherapy Combo Clinical Trial page.

Latest updates to the BMS-986226 Immunotherapy Combo Clinical Trial page
- Check2 days agoNo Change Detected
- Check9 days agoNo Change Detected
- Check16 days agoChange DetectedAdded Revision: v3.4.2; removed Revision: v3.4.1 and the government funding notice. To avoid being alerted by small changes, set an alert condition by clicking below.SummaryDifference0.4%
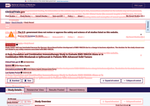
- Check23 days agoChange DetectedA government funding status notice has been added at the top of the page, and the page revision has been updated from v3.4.0 to v3.4.1.SummaryDifference0.4%
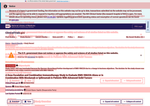
- Check30 days agoChange DetectedThe page now shows a glossary option and updates several labels, including capitalization changes in 'Last Update Submitted that Met QC Criteria' and 'No FEAR Act Data', as well as adding a new 'Revision: v3.4.0'. To avoid being alerted by small changes, set an alert condition by clicking below.SummaryDifference0.2%
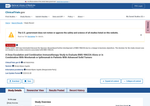
- Check44 days agoChange DetectedThe page shows an added Revision: v3.3.4 and the removal of Revision: v3.3.3; this metadata update does not affect study details, and to avoid alerts from small changes, set an alert condition by clicking below.SummaryDifference0.0%
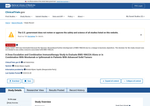
- Check66 days agoChange DetectedLocations were updated to add multiple US and Canadian sites (Massachusetts, Missouri, New Jersey, Pennsylvania, Tennessee, Alberta, Ontario). The page revision was updated to v3.3.3 and the prior location blocks were removed.SummaryDifference0.7%
- Check87 days agoChange DetectedThe page now displays Revision: v3.3.2, replacing Revision: v3.3.1. This is a minor UI/version update and does not alter study content.SummaryDifference0.0%
Stay in the know with updates to BMS-986226 Immunotherapy Combo Clinical Trial
Enter your email address, and we'll notify you when there's something new on the BMS-986226 Immunotherapy Combo Clinical Trial page.