Stay updated on Eteplirsen in DMD Patients Clinical Trial
Sign up to get notified when there's something new on the Eteplirsen in DMD Patients Clinical Trial page.
Latest updates to the Eteplirsen in DMD Patients Clinical Trial page
- Check5 days agoNo Change Detected
- Check13 days agoNo Change Detected
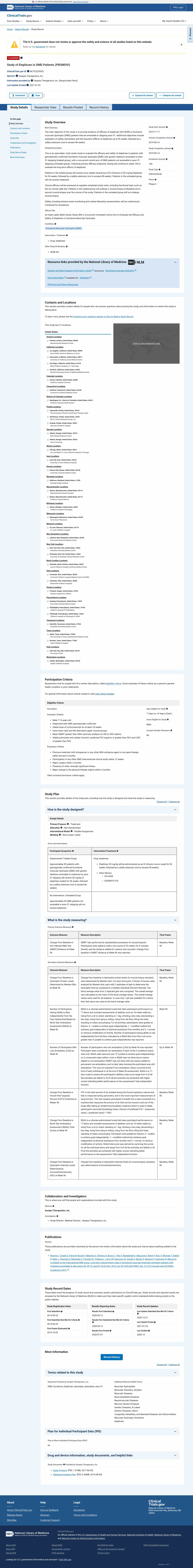
- Check20 days agoChange DetectedAdded Revision: v3.4.2. Removed the funding/operating-status notice and Revision: v3.4.1.SummaryDifference0.3%
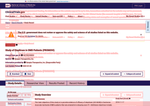
- Check27 days agoChange DetectedAdded a site-wide notice about possible information delays due to government funding and updated the site to version v3.4.1, replacing v3.4.0.SummaryDifference0.3%
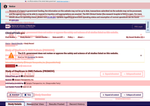
- Check34 days agoChange DetectedAdded a 'Show glossary' option and updated metadata labels to 'Last Update Submitted that Met QC Criteria' and 'Revision: v3.4.0'. Previous labels such as 'Last Update that met QC Criteria', 'No FEAR Act data', and 'Revision: v3.3.4' were removed or renamed.SummaryDifference0.2%
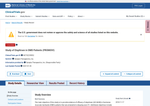
- Check48 days agoChange DetectedPage shows a software revision from v3.3.3 to v3.3.4. No changes to study details, enrollment, or locations are visible in the current context.SummaryDifference0.0%
- Check69 days agoChange DetectedAdds a full Locations section listing all study sites by state (Arizona, California, Colorado, etc.) and updates the revision to v3.3.3. It also removes the previous state-specific location blocks and the HHS Vulnerability Disclosure entry.SummaryDifference2%
- Check91 days agoChange DetectedRevision label updated from v3.3.1 to v3.3.2 in the page footer; no study content or data appear to be affected.SummaryDifference0.0%

Stay in the know with updates to Eteplirsen in DMD Patients Clinical Trial
Enter your email address, and we'll notify you when there's something new on the Eteplirsen in DMD Patients Clinical Trial page.