Stay updated on IMM-1-104 in RAS-Mutant Solid Tumors Clinical Trial
Sign up to get notified when there's something new on the IMM-1-104 in RAS-Mutant Solid Tumors Clinical Trial page.
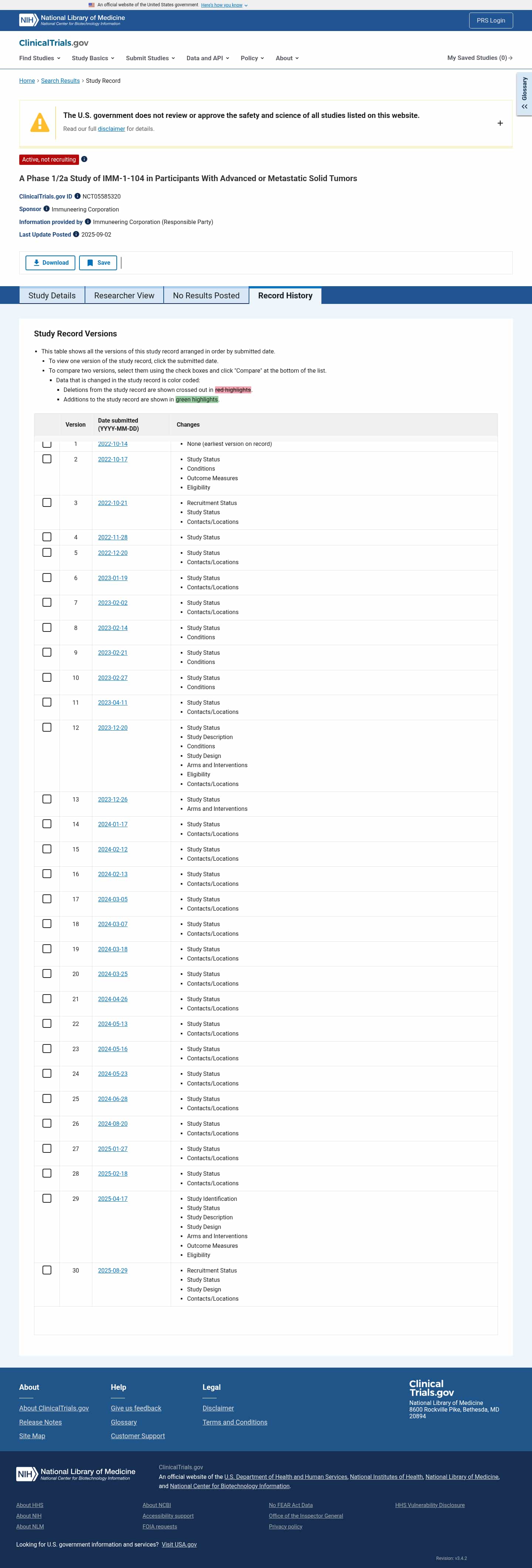
Latest updates to the IMM-1-104 in RAS-Mutant Solid Tumors Clinical Trial page
- Check6 days agoNo Change Detected
- Check13 days agoChange DetectedAdded Revision: v3.4.2 to the history and removed the prior government-funding operating-status notice. These are maintenance updates rather than changes to trial data.SummaryDifference0.6%

- Check21 days agoChange DetectedAdded a system-wide notice about a lapse in government funding and an update to the site revision (v3.4.1); the previous version (v3.4.0) was removed. To avoid being alerted by small changes, set an alert condition by clicking below.SummaryDifference0.6%
- Check28 days agoChange DetectedUI enhancements include a glossary display option and color-coded change highlights, with the revision label updated to v3.4.0. Substantive study data and content remain unchanged.SummaryDifference0.7%
- Check42 days agoChange DetectedThe page now shows a new revision entry (v3.3.4) replacing the previous v3.3.3; no substantive content changes are visible. To avoid being alerted by small changes, set an alert condition by clicking below.SummaryDifference0.1%
- Check63 days agoChange DetectedRevision: v3.3.3 was added to the history, and the HHS Vulnerability Disclosure entry along with Revision: v3.3.2 were removed. These appear to be administrative updates and do not alter study details or how users access the record.SummaryDifference0.1%
- Check92 days agoChange DetectedThe page history shows the addition of revision v3.3.2 and the removal of revision v3.2.0, indicating a routine site version update. To avoid being alerted by small changes, set an alert condition by clicking below.SummaryDifference0.1%
Stay in the know with updates to IMM-1-104 in RAS-Mutant Solid Tumors Clinical Trial
Enter your email address, and we'll notify you when there's something new on the IMM-1-104 in RAS-Mutant Solid Tumors Clinical Trial page.