Stay updated on IV ABBV-383 Combo in Multiple Myeloma Clinical Trial
Sign up to get notified when there's something new on the IV ABBV-383 Combo in Multiple Myeloma Clinical Trial page.
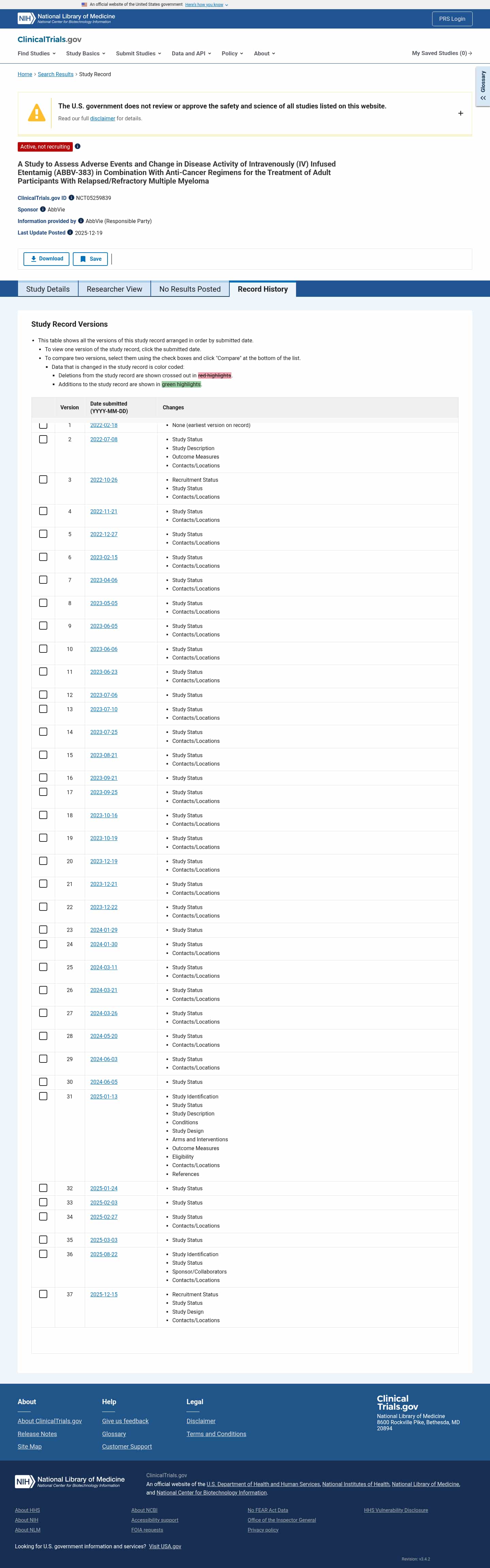
Latest updates to the IV ABBV-383 Combo in Multiple Myeloma Clinical Trial page
- Check7 days agoChange DetectedNew history entries were added to the study's Record History, displaying updates to the study status and recruitment details.SummaryDifference0.1%
- Check13 days agoChange DetectedThe history update shows the addition of Revision: v3.4.2 and the removal of the prior funding-status notice (v3.4.1), both of which are maintenance changes to page metadata. To avoid being alerted by small changes, set an alert condition by clicking below.SummaryDifference0.6%
- Check21 days agoChange DetectedA site-wide funding/status notice was added, informing users that data may not be up to date due to funding lapses. The page now shows a system revision to v3.4.1, replacing v3.4.0.SummaryDifference0.5%
- Check28 days agoChange DetectedThe record history view now includes UI clarifications: a glossary toggle and color-coded highlights (green for additions, red for deletions) with updated revision text to v3.4.0. These are cosmetic updates that do not affect substantive study information. To avoid being alerted by small changes, set an alert condition by clicking below.SummaryDifference0.7%
- Check42 days agoChange DetectedA new revision entry v3.3.4 was added to the Record History, replacing the previous v3.3.3 entry. This is a minor update to the page history and does not affect study content. To avoid being alerted by small changes, set an alert condition by clicking below.SummaryDifference0.1%
- Check64 days agoChange DetectedThe record history now shows the study status as Active, not recruiting as of 2025-12-19 and adds new sections (Contacts/Locations, Study Design, Study Status, Recruitment Status) with revision v3.3.3.SummaryDifference0.7%

Stay in the know with updates to IV ABBV-383 Combo in Multiple Myeloma Clinical Trial
Enter your email address, and we'll notify you when there's something new on the IV ABBV-383 Combo in Multiple Myeloma Clinical Trial page.