Stay updated on LGX818 and MEK162 With Third Agent in BRAF Melanoma Clinical Trial
Sign up to get notified when there's something new on the LGX818 and MEK162 With Third Agent in BRAF Melanoma Clinical Trial page.
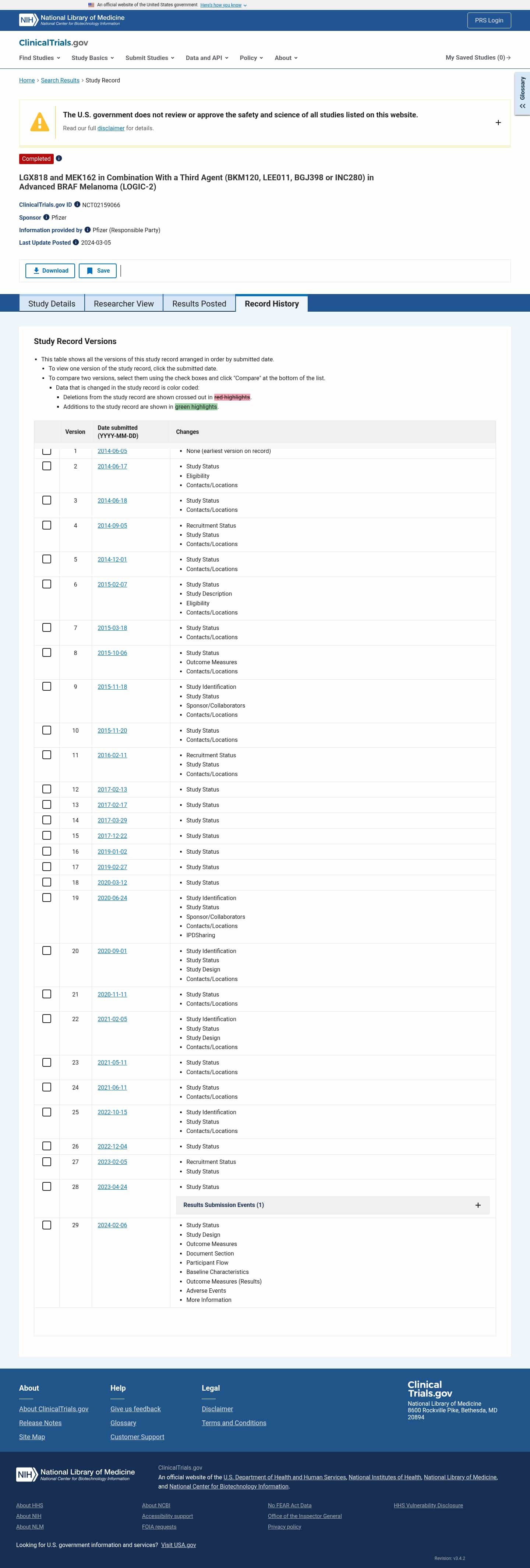
Latest updates to the LGX818 and MEK162 With Third Agent in BRAF Melanoma Clinical Trial page
- Check6 days agoChange DetectedVersion history shows updates to the Study Status, Recruitment Status, and Contacts/Locations sections, with several items added or removed. The newer version also introduces an IPD Sharing entry and other sponsor/locations edits.SummaryDifference0.1%

- Check13 days agoChange DetectedThe history shows a new revision v3.4.2 has been added; the government funding notice and the previous revision v3.4.1 were removed.SummaryDifference0.6%

- Check21 days agoChange DetectedAdded a site-wide funding notice and a new revision tag (v3.4.1), replacing the previous tag (v3.4.0). To avoid being alerted by small changes, set an alert condition by clicking below.SummaryDifference0.6%
- Check28 days agoChange DetectedThe updates are minor UI-only changes (glossary toggle, color-coded highlights, and revision/version notes) that do not alter study data or page function. To avoid being alerted by small changes, set an alert condition by clicking below.SummaryDifference0.7%

- Check42 days agoChange DetectedA new revision, v3.3.4, has been added and the previous revision, v3.3.3, removed from the page history.SummaryDifference0.1%

- Check63 days agoChange DetectedAdded Revision: v3.3.3 and removed HHS Vulnerability Disclosure and Revision: v3.3.2 from the page.SummaryDifference0.1%

Stay in the know with updates to LGX818 and MEK162 With Third Agent in BRAF Melanoma Clinical Trial
Enter your email address, and we'll notify you when there's something new on the LGX818 and MEK162 With Third Agent in BRAF Melanoma Clinical Trial page.