Stay updated on Neoadjuvant Pyrotinib in HER-2+ Breast Cancer Clinical Trial
Sign up to get notified when there's something new on the Neoadjuvant Pyrotinib in HER-2+ Breast Cancer Clinical Trial page.
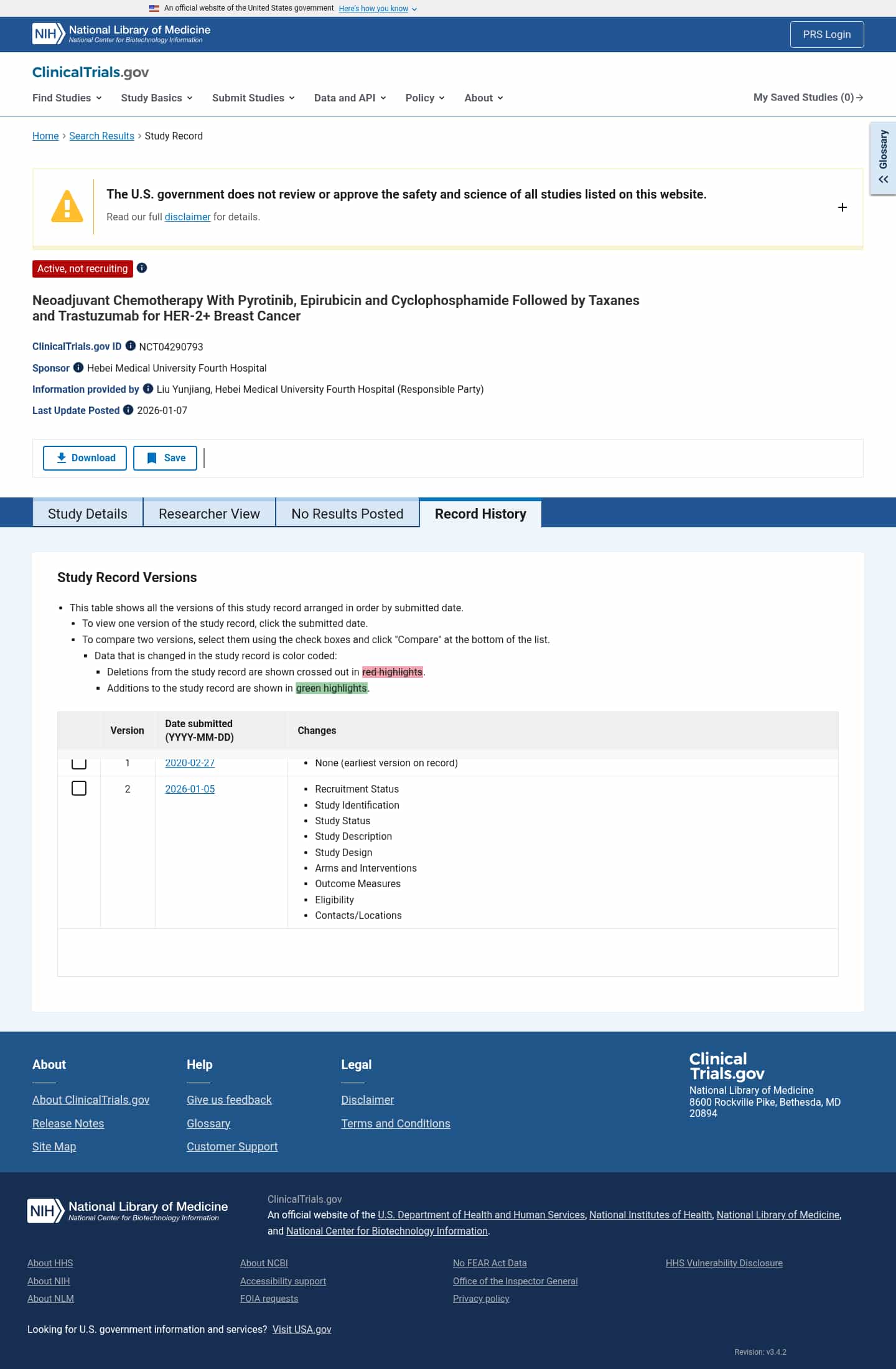
Latest updates to the Neoadjuvant Pyrotinib in HER-2+ Breast Cancer Clinical Trial page
- Check6 days agoNo Change Detected
- Check13 days agoChange DetectedAdded Revision: v3.4.2; removed the funding-lapse notice and Revision: v3.4.1.SummaryDifference0.9%

- Check20 days agoChange DetectedAdded a site-wide notice about a lapse in government funding affecting updates. Updated the page revision from v3.4.0 to v3.4.1.SummaryDifference0.9%

- Check28 days agoChange DetectedThe history page now features a Show glossary option and green/red color-coded highlights to indicate additions and deletions. It also displays the Revision: v3.4.0 release note while substantive study data remain unchanged; to avoid being alerted by small changes, set an alert condition by clicking below.SummaryDifference1%

- Check42 days agoChange DetectedActive, not recruiting as of 2026-01-07 is now shown, with new sections for Contacts/Locations, Eligibility, Outcome Measures, Arms and Interventions, Study Design, Study Description, Study Status, and Study Identification. The previous version listed Not yet recruiting and the last known status was Liu Yunjiang, Hebei Medical University Fourth Hospital.SummaryDifference2%

- Check63 days agoChange DetectedRevision: v3.3.3 was added and HHS Vulnerability Disclosure and Revision: v3.3.2 were removed from the page footer.SummaryDifference0.2%
- Check91 days agoChange DetectedThe change is a minor software version update from v3.2.0 to v3.3.2; no study data, history entries, or page content are altered. To avoid being alerted by small changes, set an alert condition by clicking below.SummaryDifference0.1%

Stay in the know with updates to Neoadjuvant Pyrotinib in HER-2+ Breast Cancer Clinical Trial
Enter your email address, and we'll notify you when there's something new on the Neoadjuvant Pyrotinib in HER-2+ Breast Cancer Clinical Trial page.