Stay updated on Safety of IV ABBV-383 in Multiple Myeloma Clinical Trial
Sign up to get notified when there's something new on the Safety of IV ABBV-383 in Multiple Myeloma Clinical Trial page.
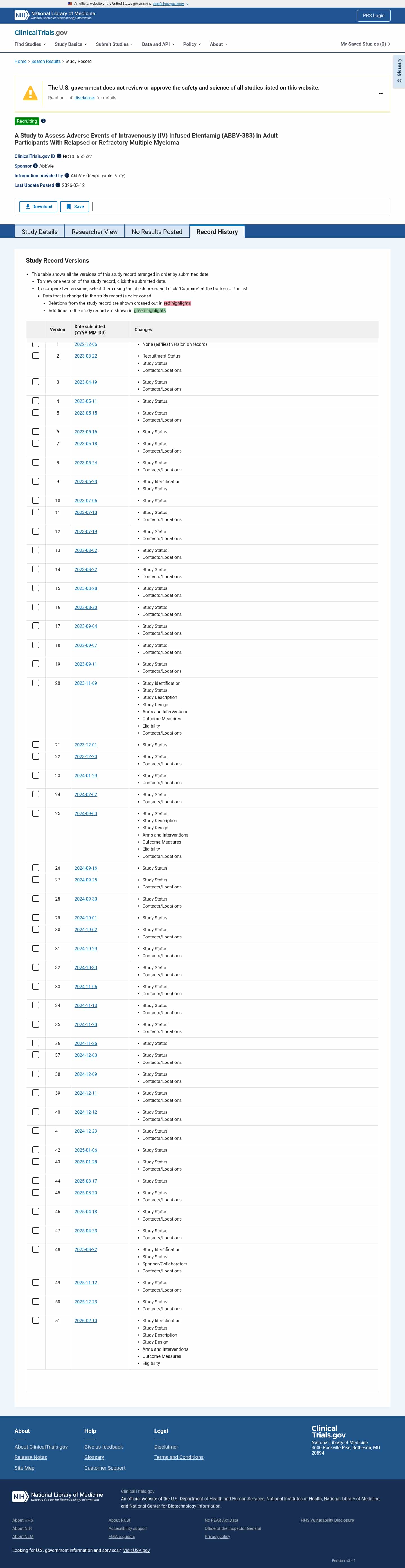
Latest updates to the Safety of IV ABBV-383 in Multiple Myeloma Clinical Trial page
- Check2 days agoChange DetectedThe study title now specifies Etentamig (ABBV-383) for intravenous use in adults with relapsed or refractory multiple myeloma, with related sections added or updated accordingly.SummaryDifference0.6%
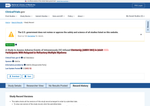
- Check9 days agoChange DetectedNew entry 'Revision: v3.4.2' was added to the record history. A general government operating status notice was removed from deletions.SummaryDifference0.4%
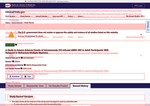
- Check16 days agoChange DetectedThe page history shows a site-wide notice about a lapse in government funding and operation status, and an update from Revision: v3.4.0 to v3.4.1; no substantive changes to the study record content were observed. To avoid being alerted by small changes, set an alert condition by clicking below.SummaryDifference0.4%
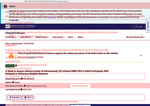
- Check23 days agoChange DetectedThe history page now uses color-coded highlights to differentiate additions and deletions and includes a revision notice (v3.4.0). A glossary toggle to show glossary terms has been added.SummaryDifference0.6%
- Check37 days agoChange DetectedThe record history shows a new revision entry (v3.3.4) added and a previous revision (v3.3.3) removed; these are minor metadata updates to the version history and do not affect core study content. To avoid being alerted by small changes, set an alert condition by clicking below.SummaryDifference0.1%
- Check52 days agoChange DetectedNew history entries for Contacts/Locations and Study Status were added (dated 2025-12-30 and 2025-12-23), and an entry from 2025-11-13 was removed.SummaryDifference0.3%
Stay in the know with updates to Safety of IV ABBV-383 in Multiple Myeloma Clinical Trial
Enter your email address, and we'll notify you when there's something new on the Safety of IV ABBV-383 in Multiple Myeloma Clinical Trial page.