Stay updated on Safety of SHR-1918 in Healthy Subjects Clinical Trial
Sign up to get notified when there's something new on the Safety of SHR-1918 in Healthy Subjects Clinical Trial page.
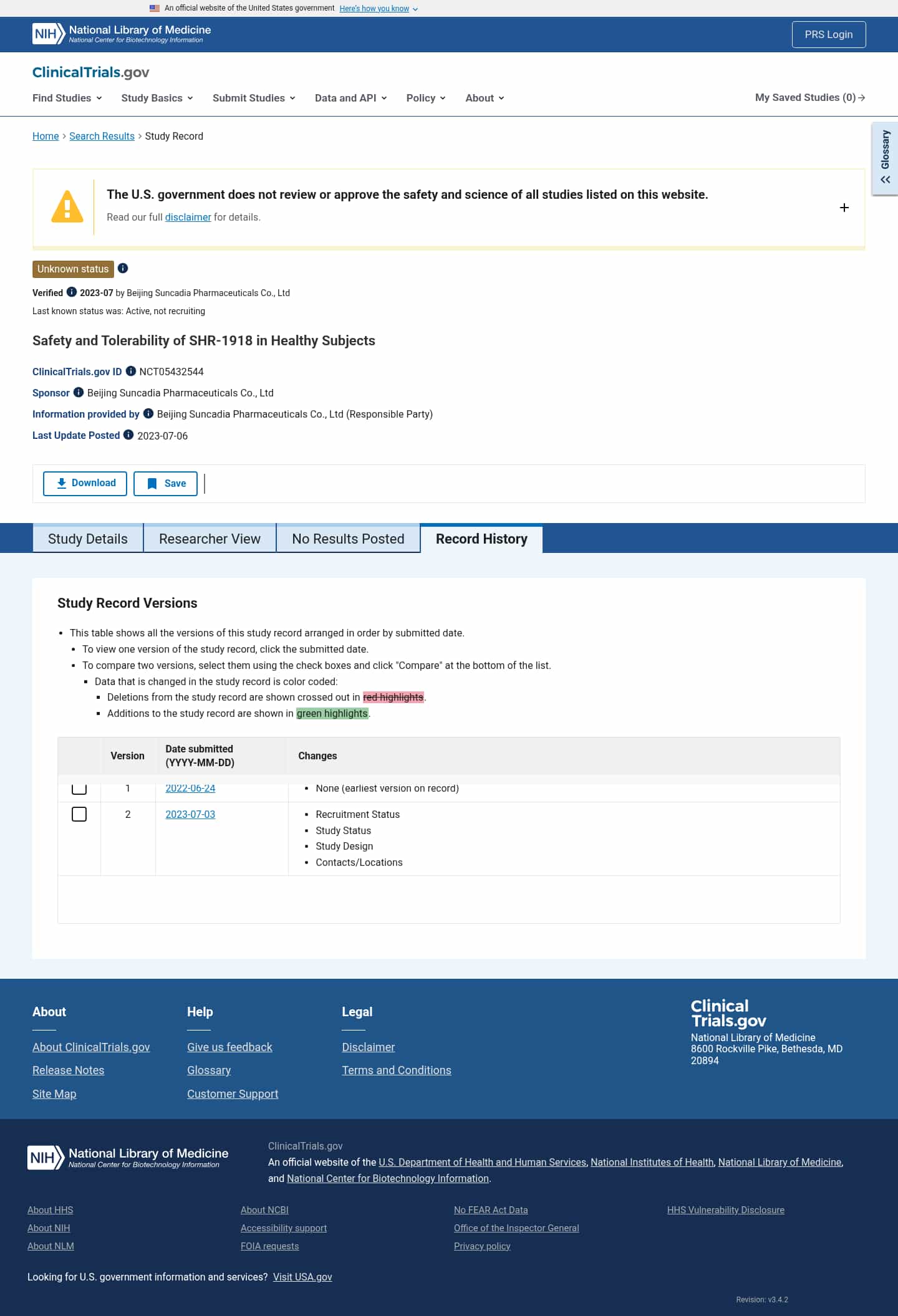
Latest updates to the Safety of SHR-1918 in Healthy Subjects Clinical Trial page
- Check4 days agoNo Change Detected
- Check11 days agoNo Change Detected
- Check18 days agoNo Change Detected
- Check25 days agoChange DetectedRevision: v3.4.2 was added. The older funding-related notice was removed and the standard government disclaimer banner is now displayed.SummaryDifference0.9%

- Check32 days agoChange DetectedAdded a government funding lapse notice regarding the NIH Clinical Center. Updated the page revision from v3.4.0 to v3.4.1.SummaryDifference0.9%
- Check39 days agoChange DetectedMinor UI updates include a Show glossary option, green additions, red deletions highlights, and revised version text (v3.4.0 vs v3.3.4) in the history view. To avoid being alerted by small changes, set an alert condition by clicking below.SummaryDifference1%
- Check53 days agoChange DetectedRevision history now includes a new entry for v3.3.4. The previous entry for v3.3.3 remains in the history.SummaryDifference0.1%
- Check75 days agoChange DetectedFooter updated to revision v3.3.3; HHS Vulnerability Disclosure was removed and the previous revision v3.3.2 was deleted.SummaryDifference0.2%
- Check96 days agoChange DetectedRecord History now includes revision v3.3.2 and removes revision v3.3.1.SummaryDifference0.1%
Stay in the know with updates to Safety of SHR-1918 in Healthy Subjects Clinical Trial
Enter your email address, and we'll notify you when there's something new on the Safety of SHR-1918 in Healthy Subjects Clinical Trial page.