Stay updated on Safety & Tolerability of MRT5005 in CF Adults Clinical Trial
Sign up to get notified when there's something new on the Safety & Tolerability of MRT5005 in CF Adults Clinical Trial page.
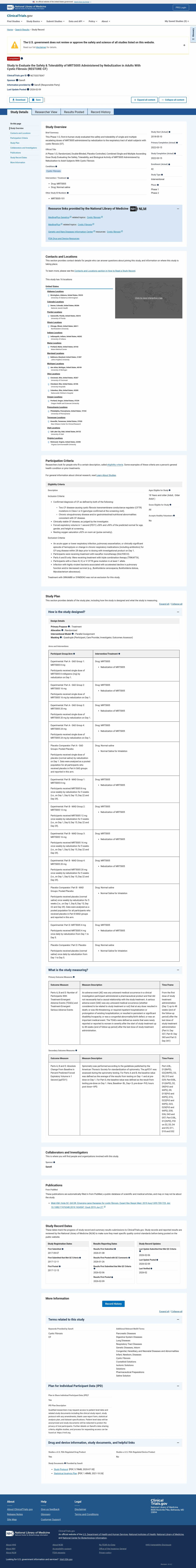
Latest updates to the Safety & Tolerability of MRT5005 in CF Adults Clinical Trial page
- Check7 days agoNo Change Detected
- Check14 days agoNo Change Detected
- Check21 days agoChange DetectedRESTORE-CF (NCT03375047) page updated to include Results Posted (Sanofi, 2026-02-09), 16 trial locations, and expanded details on study arms, dosing schedules, and primary/secondary outcomes.SummaryDifference7%
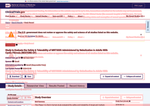
- Check28 days agoChange DetectedA site-wide funding-status notice now appears, indicating information may not be up to date due to a lapse in government funding. The footer revision tag has been updated from v3.4.0 to v3.4.1.SummaryDifference0.4%
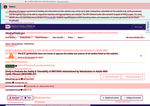
- Check35 days agoChange DetectedAdded a 'Show glossary' option and updated metadata to include 'Last Update Submitted that Met QC Criteria', 'No FEAR Act Data', and a new revision 'v3.4.0'. Removed several legacy labels/fields such as 'Last known status was:' and sponsor-related text ('Translate Bio, Inc.','by','2020-11','Verified','Unknown status'), indicating cleanup of non-critical page content.SummaryDifference0.5%
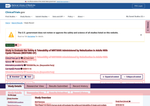
- Check49 days agoChange DetectedResults Submitted and Revision: v3.3.4 were added, replacing the previous No Results Posted and Revision: v3.3.3.SummaryDifference0.1%
- Check71 days agoChange DetectedAdded a unified Locations section listing participating states (Alabama, Colorado, Florida, Illinois, Indiana, Maine, Maryland, Michigan, Ohio, Oregon, Pennsylvania, Tennessee, Utah, Virginia) and corresponding entries. The previous state-specific location groupings (Alabama Locations, Colorado Locations, etc.) were removed.SummaryDifference1%
- Check100 days agoChange DetectedA new sentence explains that publications shown are automatically filled from PubMed and may not all be related to the study, and the revision notice updates from v3.2.0 to v3.3.2. To avoid being alerted by small changes, set an alert condition by clicking below.SummaryDifference0.1%
Stay in the know with updates to Safety & Tolerability of MRT5005 in CF Adults Clinical Trial
Enter your email address, and we'll notify you when there's something new on the Safety & Tolerability of MRT5005 in CF Adults Clinical Trial page.