Stay updated on Switching to B/F/TAF in Virologically Suppressed Adults Clinical Trial
Sign up to get notified when there's something new on the Switching to B/F/TAF in Virologically Suppressed Adults Clinical Trial page.
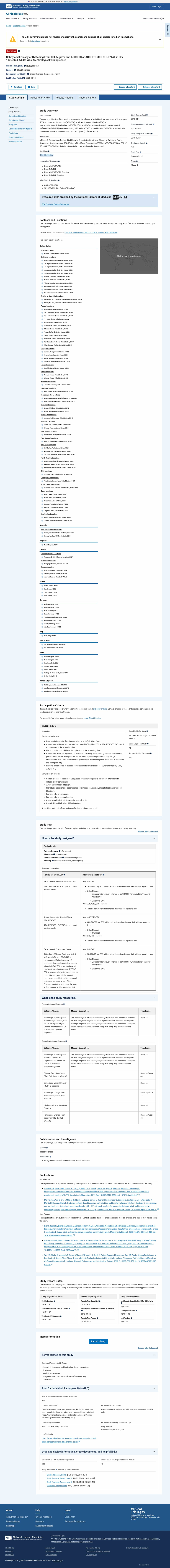
Latest updates to the Switching to B/F/TAF in Virologically Suppressed Adults Clinical Trial page
- Check3 days agoNo Change Detected
- Check10 days agoChange DetectedAdded Revision: v3.4.2 and removed Revision: v3.4.1 in the revision notes. To avoid being alerted by small changes, set an alert condition by clicking below.SummaryDifference0.0%

- Check18 days agoChange DetectedAdded a new publication citation (Andreatta et al., J Antimicrob Chemother, 2019) with revision 3.4.1, and removed the Erratum reference for the same article (revision 3.4.0). To avoid being alerted by small changes, set an alert condition by clicking below.SummaryDifference0.1%

- Check25 days agoChange DetectedThe new screenshot shows the Study Details page with no additions or deletions to core content; the information on eligibility, endpoints, locations, and timeframes remains unchanged from the previous version. To avoid being alerted by small changes, set an alert condition by clicking below.SummaryDifference0.0%

- Check32 days agoChange DetectedUI and metadata updates include a glossary toggle, the addition of 'Last Update Submitted that Met QC Criteria' and 'No FEAR Act Data' notes, and the revision label updated to v3.4.0 (replacing the older v3.3.4 variant).SummaryDifference0.1%
- Check39 days agoChange DetectedRevision: v3.3.4 was added and Revision: v3.3.3 was removed, indicating a minor site update with no observed changes to the study record. To avoid being alerted by small changes, set an alert condition by clicking below.SummaryDifference0.0%

- Check46 days agoChange DetectedThe Publications entry for Andreatta et al. 2019 was updated to include the Erratum (3646-3647) and the DOI 10.1093/jac/dkz412, replacing the previous citation. This update does not affect study results or methods.SummaryDifference0.0%

Stay in the know with updates to Switching to B/F/TAF in Virologically Suppressed Adults Clinical Trial
Enter your email address, and we'll notify you when there's something new on the Switching to B/F/TAF in Virologically Suppressed Adults Clinical Trial page.