Stay updated on Ziltivekimab vs Placebo in Heart Failure & Inflammation Clinical Trial
Sign up to get notified when there's something new on the Ziltivekimab vs Placebo in Heart Failure & Inflammation Clinical Trial page.
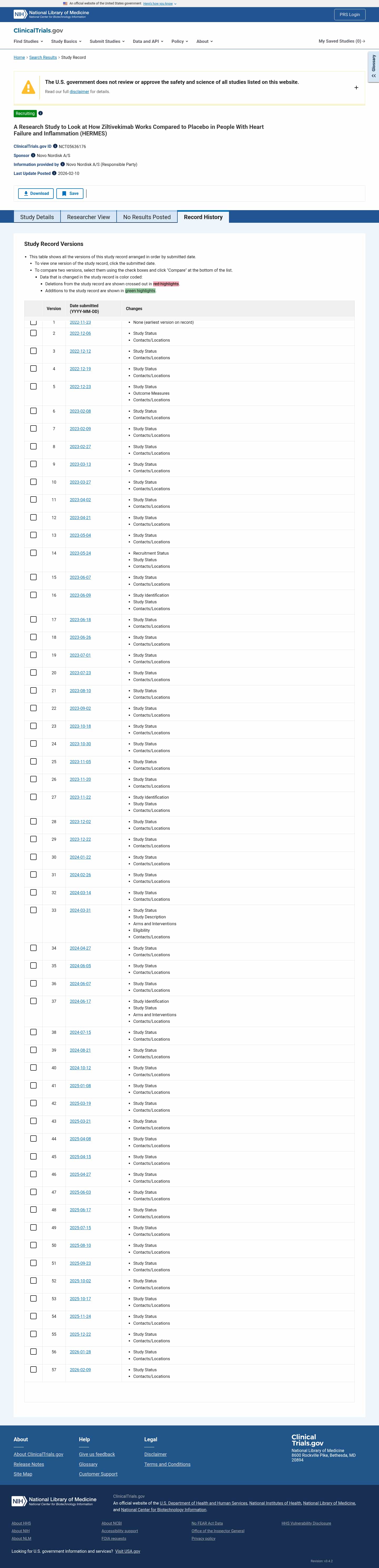
Latest updates to the Ziltivekimab vs Placebo in Heart Failure & Inflammation Clinical Trial page
- Check5 days agoNo Change Detected
- Check12 days agoChange DetectedThe history now shows new version entries (2026-02-09 and 2026-02-10) and a revision label, and it removes an older funding notice; these are administrative record-keeping updates and do not change the trial details. To avoid being alerted by small changes, set an alert condition by clicking below.SummaryDifference0.7%
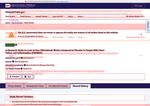
- Check19 days agoChange DetectedA site-wide funding notice was added, and new revision history entries (v3.4.1) with updated Contacts/Locations and Study Status appeared dated 2026-01-28 and 2026-01-29. The prior revision (v3.4.0) dated 2025-12-23 was removed.SummaryDifference0.7%
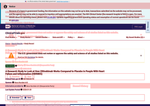
- Check26 days agoChange DetectedGlossary toggle and color-coded change highlights (green for additions, red for deletions) were added. Revision metadata was updated to v3.4.0 and the 'No FEAR Act Data' label appeared, replacing the older 'No FEAR Act data' and 'Revision: v3.3.4' entries.SummaryDifference0.5%
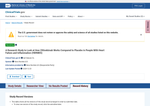
- Check40 days agoChange DetectedThe Record History now shows Revision: v3.3.4 as the current version, replacing Revision: v3.3.3. This is a minor metadata update to the page label and does not affect study data or overall functionality.SummaryDifference0.1%
- Check55 days agoChange DetectedAdded Contacts/Locations and Study Status in the latest update, signaling updated trial details and contact information. An earlier version from 2025-11-25 was deleted.SummaryDifference0.3%
- Check62 days agoChange DetectedFooter area updated to show Revision: v3.3.3, and HHS Vulnerability Disclosure and Revision: v3.3.2 were removed. No study data, statuses, or history content were changed; To avoid being alerted by small changes, set an alert condition by clicking below.SummaryDifference0.1%
Stay in the know with updates to Ziltivekimab vs Placebo in Heart Failure & Inflammation Clinical Trial
Enter your email address, and we'll notify you when there's something new on the Ziltivekimab vs Placebo in Heart Failure & Inflammation Clinical Trial page.