What Is Regulatory Intelligence in the Pharmaceutical Industry?
By Emily Fenton
Updated April 14, 2025

Regulatory Intelligence in the Pharmaceutical Industry: The State of the Industry 2025
Introduction
Regulatory intelligence is a broad term that varies from industry to industry. But, in most cases, it involves a three-step strategy:
- Monitoring information about changes to relevant laws and regulations;
- Analyzing data;
- Leveraging the data analysis to create a targeted action plan to ensure compliance.
All industries must maintain regulatory compliance. While the life sciences industry is no exception, pharmaceutical companies are particularly under pressure to monitor the changes in the regulated landscape, and respond accordingly.
Increasing complexities surrounding variables like product development, clinical trials and technological innovations make the pharmaceutical industry one of the most heavily regulated.
Fortunately, the rise of regulatory tracking software, like Visualping, make it easier to monitor and respond to regulatory changes in the pharmaceutical industry.
Regulatory Intelligence in Pharmaceuticals
Regulatory intelligence in the pharmaceutical industry requires Pharma and Biotech companies, as well as their internal lawyers, part of their legal compliance team, to understand relevant regulations. They also need to be aware of trends, players, bureaucracies and regulatory forces that drive key developments in the industry.
Once having mapped the regulatory landscape, practitioners can make strategic and informed decisions to minimize regulatory risks, and maintain compliance.
Importance of Maintaining Pharmaceutical Compliance
The pharmaceutical industry is one of the most heavily scrutinized and regulated sectors worldwide. Pharmaceuticals can have devastating health effects if not thoroughly researched and monitored for quality and safety.
Although it takes work to maintain pharmaceutical compliance, the effort is well worth it. With a strong understanding of the legal requirements expected of them, companies empower themselves to avoid hazards, and develop proper plans and protocols to mitigate compliance risk.
Some of the risks of not monitoring regulatory changes and staying compliant with pharmaceutical regulations include large fines, lawsuits, damage to your brand and reputation, and business closure. Staying on top of the latest compliance requirements, then, is a massive undertaking, but with a big payoff.
Regulatory Intelligence Professionals in the Industry
Because regulatory compliance is so critical in the pharmaceutical industry, most companies have specialized departments or teams focused on both regulatory intelligence and compliance management. This is often referred to as regulatory change management.
These teams are charged with learning about external compliance requirements, and staying up to date on all changes -- these days, usually with the help of legislative tracking software. When critical developments occur, this team shares information, as needed, with the appropriate stakeholders in the organization.
These departments vary from company to company, but they usually include team members specialized in legal and regulatory compliance. Larger companies usually have dedicated teams with multiple full-time employees whose jobs revolve around compliance.
Teams at Large vs Small Companies
Smaller companies, on the other hand, have to balance regulatory intelligence with other job functions. For example, some companies assign responsibility for regulatory compliance to the operations manager, who must monitor the external regulatory landscape, and oversee compliance concerns along with their other duties.
Larger pharma companies have a leg up on smaller companies in this way since they have the resources and personnel to specialize in compliance intelligence. These companies are better poised to cultivate active regulatory intelligence vs. passive regulatory intelligence.
Roles and Responsibilities
What are some of the roles and responsibilities involved in regulatory intelligence? Some of the key duties of regulatory intelligence professionals in the pharmaceuticals sector include:
- Researching emerging compliance trends and patterns
- Analyzing regulations and interpreting them for the rest of the team
- Creating detailed reports on current, changing, and new regulations
- Consulting with the team or serving as an advisor in other areas of the company
- Developing strategies for company-wide regulatory compliance
- Developing and executing compliance training where needed
Regulatory intelligence analysts are responsible for researching information about new or evolving regulatory activity.
For example, suppose new legislation is passed. The analysts are responsible for knowing when those updates occur, and disseminating that information into both helpful insights and actionable recommendations for the rest of the organization.
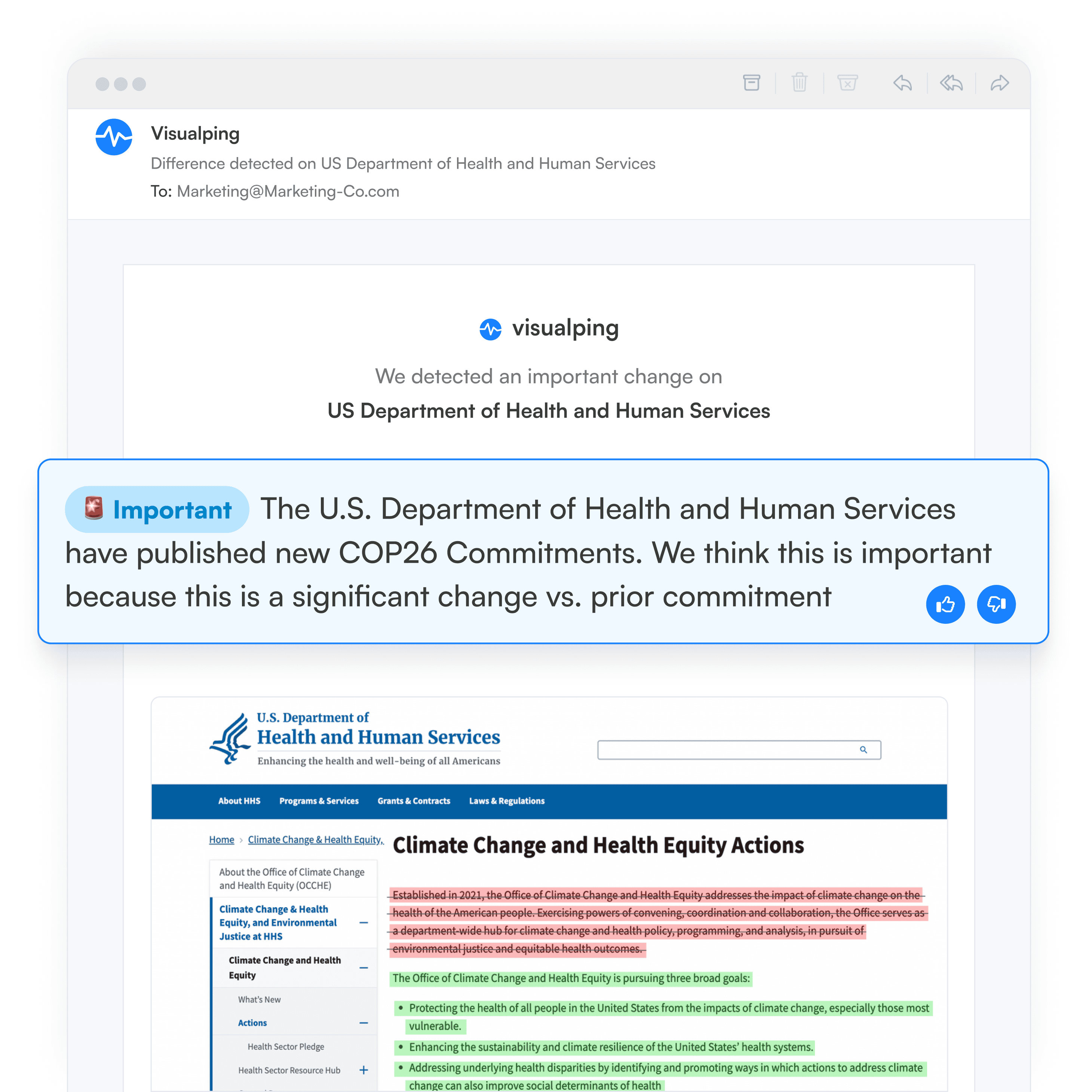
Rather than manually rechecking various government sources for potential updates, compliance teams can use website change monitoring tools, like Visualping, to get alerts when updates occur. With Visualping, the alerts include AI-generated summaries of the change, as well as a screenshot, with the changes highlighted.

Active vs. Passive Regulatory Intelligence
There are two main approaches to regulatory intelligence: an active approach and a passive approach.
Passive Regulatory Intelligence
Passive regulatory intelligence involves:
- Monitoring
- Analyzing
- Disseminating
These three actions apply to regulatory information that is generally available to the public at large.
Passive regulatory intelligence is a less targeted approach that not only takes less time but also doesn’t require a great deal of specialized knowledge. This is because the information in question is not confidential but available to whoever wants to access it.
The passive approach may be practical for smaller companies or for employees who are wearing multiple compliance hats. It’s generally best suited for broader compliance trends as well as potential risks or new developments, such as changing legislation.
To summarize, these are some of the benefits of the passive approach to regulatory intelligence:
- Little to no specialized knowledge needed
- The ability to identify broad compliance trends
Some potential risks or cons of the passive approach include:
- Less targeted compared to the active approach
- Relies heavily on publicly available information
- Demands a continuous cycle of data collection, analysis, and information dissemination
The passive approach doesn’t work for every company, at least not on its own. In some cases, it may be better to use the active approach or a mix of the two approaches.
Active Regulatory Intelligence
The active approach involves:
- Collecting information, whether confidential, nonpublic, or public
- Leveraging niche industry knowledge
- Leveraging targeted connections and professional relationships
Although the active approach may involve collecting public information, it typically includes processes associated with discovering and collecting confidential information as well. Because the information is confidential, only someone with specialized knowledge or targeted connections in the industry can access it.
In order to successfully executive an active approach to regulatory intelligence, one must have:
- Solid contacts, usually with major players within the industry
- The ability to properly conduct strategic interviews
- Knowledge of how to handle confidential documents
- Knowledge of how to manage sources
- In some cases, surveillance skills
Organizations benefit from the active approach when they need specific information about a situation, target organization, or regulatory developments.
To summarize, some of the benefits of the active approach include:
- Gathering information specific to the needs of an organization
- Targeted, niche intelligence
- The ability to detect regulatory developments sooner than competitors
Some of the cons of the active approach are that this approach requires specialized skills and access to major players in the industry. In addition, the data collection process involves interviews, surveillance, and other strategies that make it more time-consuming and demanding.
The Top Regulatory Intelligence Tool for the Pharmaceutical Industry
Visualping
Laws and regulations regarding pharmaceuticals are very complex, and evolve at a rapid pace -- making compliance in the industry even more challenging.
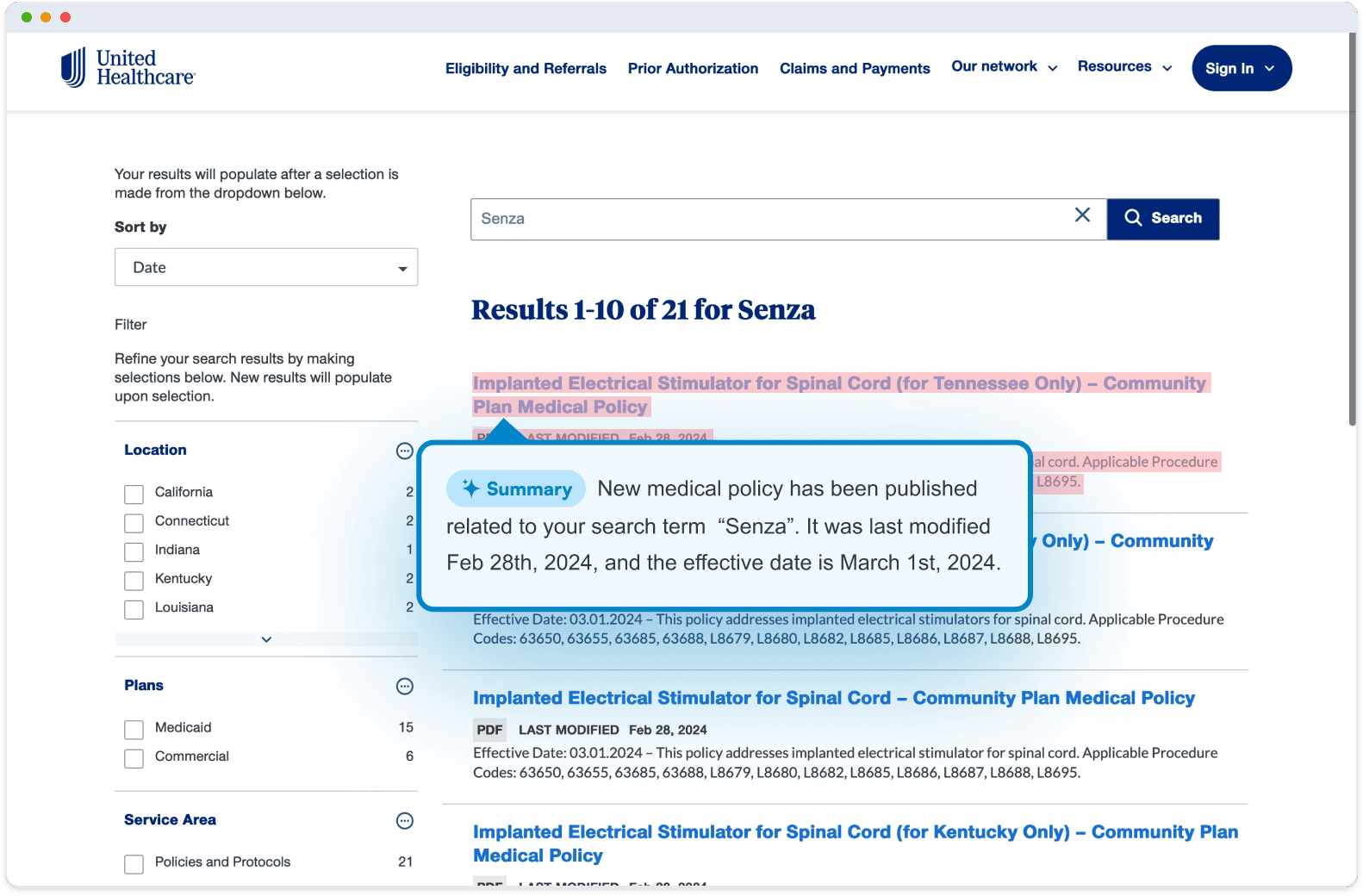
Visualping is an AI website monitoring tool that is often used to track regulatory changes for companies of all sizes.
When there's an update online, on one of the web sources you're monitoring, Visualping sends you and your team an alert, with an AI-generated summary of the change, distilled in two to three lines. The alert also includes a screenshot of the page, with the changes highlighted.
Alerts can be sent through email, or popular messaging apps, like Slack or Microsoft Teams.
Easy-to-Use
Visualping](https://visualping.io/business/) is easy to use. You can track any web page you want on the internet for the legal and regulatory updates you're after -- like updates to state and federal bills and debate, regulatory agency announcements and news articles. You can monitor an entire web page for changes, or select parts of the page you care about.
To get started, simply copy and paste the URL of the page you're interested in.
You set the frequency at which Visualping will check the web page. Get real-time change alerts, or monitor a page every 3 hours, 6 hours, daily -- it's really up to you.
It offers powerful customization features, but you don’t need to be a tech whiz to use it. All you need to do is select the sites you need to monitor, decide how often you want the tool to check them, and provide an email address to receive alerts.
More Intelligence, Less Noise
The AI summaries, included in all the alerts, makes it easy to quickly understand the update, so you can determine whether it's relevant to you or not.
With the keyword alerts feature, you can filter through the noise and only get notified when a certain word or phrase you're interested in gets added to the page.
Quickly understand page changes
Regulatory updates are easy to understand with Visualping. Simply open your email alert to see what's changed with before-and-after text snippets and screenshots. In your user dashboard, you can go back and review changes, to analyze updates over time.
You can even generate historical change reports across monitored pages, to view many updates in a glance.
Amplify Regulatory Intelligence with Visualping
Regulatory intelligence is an important strategy every pharma or biotech company should use in order to stay compliant and ahead of their competition. Fortunately, Visualping makes staying up to date on compliance changes easier than ever. Get started with Visualping today.
Get regulatory updates -- straight from the source.
Sign up with Visualping to get alerted of regulatory changes from anywhere online.
Emily Fenton
Emily is the Product Marketing Manager at Visualping. She has a degree in English Literature and a Masters in Management. When she’s not researching and writing about all things Visualping, she loves exploring new restaurants, playing guitar and petting her cats.